HYDROGEN PRODUCTION BY PHOTOELECTROLYSIS
By Robert Huang
Abstract
Hydrogen will power future fuel cell vehicles and eliminate traffic related pollution. However, hydrogen does not exist naturally and must be extracted from other compounds such as fossil fuels, biomass, and water. Current methods of hydrogen production consume fossil fuels and emit greenhouse gases. Photoelectrolysis, which is still in experimental stages, is a renewable method of hydrogen production. A photoelectrode is a semiconductor device that absorbs solar energy and generates the necessary voltage to split water molecules. It consists of photovoltaic, catalytic, and protective layers. The University of Hawaii at Manoa has developed a low-cost photoelectrode that has a solar-to-hydrogen efficiency rate of 10%. Photoelectrolysis is the most efficient method of renewable hydrogen production. Photoelectrolysis offers a promising long-term solution for supplying the future hydrogen economy.
HYDROGEN PRODUCTION BY PHOTOELECTROLYSIS
The United States Department of Energy has determined hydrogen to be energy carrier of the future. Because fuel cells can generate electricity from hydrogen and oxygen, while only emitting water, future fuel cell vehicles will eliminate traffic-related pollution and reduce fossil fuel consumption. However, pure hydrogen does not exist naturally in high concentrations on Earth. Instead, hydrogen must be extracted from other compounds such as water, biomass, and fossil fuels.
Current methods of hydrogen production are not adequate to support the future hydrogen economy. Steam methane reforming accounts for 95% of the United States’ hydrogen production (6:13). This process extracts hydrogen and carbon dioxide from natural gas. However, steam methane reforming is not a viable long-term method of hydrogen production because natural gas is not renewable and carbon dioxide is a major greenhouse gas. Another method of hydrogen production is water electrolysis. Water electrolyzers use electricity to split water molecules into hydrogen and oxygen. The problem with electrolysis is that electricity is still mainly generated by burning fossil fuels. Electrolyzers can be driven by renewable sources of electricity such as solar or wind powered generators, but such a system would be expensive and inefficient.
A promising future method of hydrogen production is photoelectrolysis, which uses solar energy to extract hydrogen directly from water. Photoelectrolysis integrates solar energy collection and water electrolysis into a single photoelectrode. This device eliminates the need for a separate power generator and electrolyzer, reducing overall
costs and increasing efficiency. Photoelectrolysis systems are still in the experimental stage. However, photoelectrolysis will become an important means to future hydrogen production because it is powered by renewable solar energy. This paper will explain the theory of photoelectrolysis, photoelectrode design, and the role of photoelectrolysis in the future hydrogen economy.

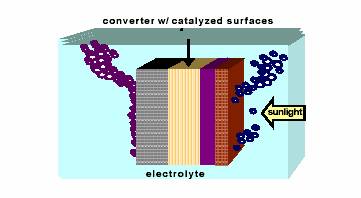
Figure 1. Generic hydrogen photoelectrode
(Source: Miller, Eric and Rocheleau, Richard. Photoelectrochemical Production of Hydrogen, University of Hawaii at Manoa: Hawaii Natural Energy Institute, 2002, p.2.)

Figure 2. Picture of a working photoelectrode
(Source: Production and Delivery of Hydrogen, Hydrogen Fuel Cells & Infrastructure Program, US Department of Energy. http://www.eere.energy.gov/hydrogenandfuelcells/hydrogen/production.html#photolytic)
1.0 Theory of Photoelectrolysis
Photoelectrolysis uses photoelectrochemical (PEC) light collecting systems to power the electrolysis of water. When exposed to sunlight, a semiconductor photoelectrode, submerged in an aqueous electrolyte, will generate sufficient electrical energy to promote hydrogen and oxygen evolution reactions (HER and OER). The HER releases electrons into the electrolyte, while the OER requires free electrons. Depending on the type of semiconductor material and the solar intensity, the produced current density is 10-30 mA/cm2. At these current densities, the voltage required for electrolysis is approximately 1.35 V (8:4).
For direct photoelectrochemical decomposition of water to occur, several requirements must be met. The energy band of the semiconductor materials must overlap the energy levels of the hydrogen and oxygen reduction reactions. The semiconductor system must be stable under photoelectrolysis conditions. Finally, the charge transfer from the surface of the semiconductor must be fast enough to prevent corrosion and also reduce energy losses due to overvoltage.
Photoelectrolysis performance is measured by solar-to-hydrogen (STH) conversion efficiency. The STH rate is based on the following formula:
![]() (1)
(1)
where:
![]()
JT = hydrogen current density
The theoretical maximum STH conversion efficiency for photoelectrolysis is 42%. However, current photoelectrolysis systems only have 8%-14% efficiency rates (7:1).
2.0 Photoelectrode Components
A photoelectrode has photovoltaic, catalytic, and protective layers, which can be modeled as separate components. Each layer affects the overall solar-to-hydrogen efficiency the photoelectrochemical system.
The photovoltaic layer is made of light-absorbing semiconductor material such as titanium dioxide or gallium/arsenide. The light absorption of the semiconductor material is directly proportional to the performance of the photoelectrode. Semiconductors with wide band gaps provide the necessary potential for water splitting. However, because they absorb only the most energetic portion of the solar spectrum, STH conversion efficiencies are limited. Light absorption can be increased by modification of the semiconductor materials, or by the addition photo-sensitizers, such as dyes that absorb a larger portion of the solar spectrum (3:7).
The catalytic layers of PEC cells also affect electrolysis performance. PEC systems require appropriate catalysts to enhance the water splitting reactions. The HER catalyst will generate hydrogen when electrons are released into the aqueous electrolyte. The OER catalyst will generate oxygen as electrons return from the aqueous electrolyte. The OER requires greater overvoltage than the HER.
The encapsulant layer is another important component of the photoelectrode. This protective layer prevents the semiconductor from corroding within the aqueous electrolyte. This layer must be highly transparent to allow maximum solar energy to reach photovoltaic semiconductor layer.

Figure 3. Diagram of photoelectrode components
(Source: Miller, Eric and Rocheleau, Richard. Photoelectrochemical Production of Hydrogen, University of Hawaii at Manoa: Hawaii Natural Energy Institute, 2002, p. 4.)
3.0 Potential Photoelectrode Design
A potentially low cost, high efficiency PEC system has been developed at the University of Hawaii at Manoa. The photoelectrode consists of semiconductor, catalytic, and protective thin-films deposited on a stainless steel substrate. The semiconductor layer uses high efficiency copper-indium-gallium-diselenide (CIGS) photoconverter junctions to produce electricity from solar energy. Conductive indium-tin oxide (ITO) film transfers generated current to a nickel molybdenum (NiMo) layer, which acts as a HER catalyst. The semiconductor layer is covered by a transparent encapsulant, which prevents corrosion from the aqueous electrolyte. An iron nickel-oxide (Fe:NiOx) layer, which acts as an OER catalyst, is deposited onto the back of the stainless steel substrate. A diagram of the photoelectrode is shown below.
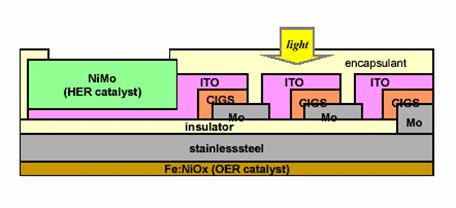
Figure 4. CIGS photoelectrode layout
(Source: Miller, Eric and Rocheleau, Richard. Photoelectrochemical Production of Hydrogen, University of Hawaii at Manoa: Hawaii Natural Energy Institute, 2002, p.5.)
This design has a few disadvantages. Electrical current collects laterally along the ITO layers, decreasing STH efficiency. Also, the small surface area of the NiMo layer degrades hydrogen conversion performance. However, the small HER catalyst surface area is slightly offset by the greater overpotential of the OER. With these current design
flaws, the CIGS photoelectrode has a solar-to-hydrogen efficiency of just over 10%, which meets the Department of Energy’s efficiency goals for a low cost PEC system.
4.0 Role of Photoelectrolysis in the Hydrogen Economy
During the State of the Union Address, President Bush announced his Freedom CAR and Fuel initiative, which will provide $1.2 billion of funding for hydrogen fuel cell vehicle and hydrogen production research. Under the initiative, fuel cell vehicles are targeted to become cost competitive after 2010. The United States currently produces nine million tons of hydrogen per year for chemical production, petroleum refining, metals treating, and electrical applications. An estimated 40 million tons of hydrogen will be required annually to fuel 100 thousand fuel cell vehicles (6: 11).
Photoelectrolysis is the cheapest and most efficient method of renewable hydrogen production. The current cost of hydrogen production by photoelectrolysis is estimated to be $41.30 per Gigajoule (7:1).
However, nonrenewable hydrogen production methods are currently cheaper than photoelectrolysis. With current natural gas prices, SMR hydrogen production costs are only $7.00 per Gigajoule (1:3). With current grid electricity prices, electrolyzers produce hydrogen at a cost of $24.00 per Gigajoule (2:13). However, SMR and electrolyzer hydrogen production costs are expected to increase along with the price of fossil fuels. On the other hand, photoelectrolysis costs will continue to decrease due to photoelectrode solar-to-hydrogen efficiency gains.
Because hydrogen fuel cell vehicles are not expected to be sold to the public until after 2010, much time remains for photoelectrolysis research. By then the hydrogen production costs difference between photoelectrolysis and nonrenewable
hydrogen production methods will greatly diminish. Because of this, photoelectrolysis and future renewable processes will become the long-term means of hydrogen production.
Photoelectrolysis is currently the cheapest and most efficient method of renewable hydrogen production. Photoelectrolysis integrates solar energy harvesting and water electrolysis into a semiconductor photoelectrode. This technology is still in experimental stages, but it already demonstrates promising efficiency rates and hydrogen production costs. Nonrenewable hydrogen production methods will only serve as a short-term supply for the hydrogen economy because they offset the benefits of fuel cell vehicles by consuming fossil fuels and emitting greenhouse gases. Research funding for photoelectrolysis and other renewable hydrogen production methods is essential for the emergence of a future hydrogen economy.
References
1. Bollinger, Robert B. Low Cost Hydrogen Production Platform. Praxair Inc. p. 3.
2. Goswami, D. Yogi. Et. Al. Task 4: Hydrogen Production. University of Florida, Department of Aerospace and Mechanical Engineering. 1999. p. 13.
3. IEA Agreement on the Production and Utilization of Hydrogen 1999 Annual Report.
International Energy Agency. 1999.
4. Mann, Jeremiah. Investigating the Band Gap and Flat Band Potential of Fe2O3 Doped
with Ti, Ta, Sn and Zn. DOE Native American and Student Internship Program, University of Santa Cruz, National Renewable Energy Laboratory. 1998. pp. 6-8.
5. Miller, Eric and Rocheleau, Richard. Photoelectrochemical Production of Hydrogen, University of Hawaii at Manoa: Hawaii Natural Energy Institute, 2002. pp. 1-6.
6. National Hydrogen Energy Roadmap. United States Department of Energy, 2002. pp. 13-18.
7. Production and Delivery of Hydrogen, Hydrogen Fuel Cells & Infrastructure Program, US Depertment Energy. <http://www.eere.energy.gov/hydrogenandfuelcells/hydrogen/production.html>
8. Warren, Scott. Photoelectrochemical Splitting of Water: Using Porphyrins as Catalysts. DOE ERULF Program, Whitman College, National Renewable Energy Laborotory. 2000. p. 4.